The 500mL glass graduated cylinder is our second largest & comes with numbered markings at every 50mL Sub gradations allow for measuring accuracy of 5mL It comes with a hex base for stability & is made from heat resistant glass Click on the Tabs below for specifications and best use practices Check out our blog post "How to Read a Graduated Cylinder" Note Marking styleTo have of 05°C and of 1°C The barometer was determined to have of 033 kPa and negligible The overall uncertainty, , was then calculated for each piece of equipment using Equation 1 as seen below (1) The overall uncertainty for the digital manometer, 250 mL graduated cylinder, 1000 mL graduated cylinder, thermometer, and barometer were determined to be the following 041 kPa, 224 mLFlared top rims & large spouts for pouring Hexagonshaped bases provide stability Nonwetting surface eliminates concave meniscus Autoclavable at 121°C Graduations 10mL Dimensions 24" Dia (26" at spout) x 177" Hgt
Blogs Nvcc Edu Alchm Files 16 08 111 02densityandmeasurementsfall16 Pdf
How to read a 1000 ml graduated cylinder
How to read a 1000 ml graduated cylinder-= When using a 50mL graduated cylinder to measure 15 mL, the absolute uncertainty is ±1% of 50 mL, or ±050 mL and the relative uncertainty is % 15 05 0 100 33Perfect measurement Even when using expensive lab equipment there some degree of uncertainty in measurement The general rule of thumb is you can estimate one more digit past the smallest division on the measuring device If you look at a 10mL graduated cylinder, for example, the smallest graduation is tenth of a milliliter (01mL)




Pyrex 1000 250 Glass Beaker 250ml
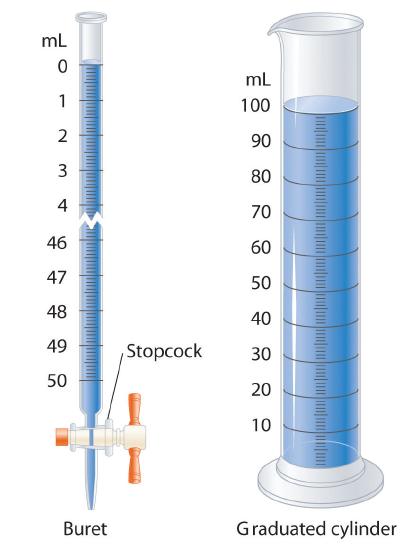
In the graduated cylinder shown in Figure 1, the mL graduations are marked and can be read with certainty The uncertainty value for this volume graduated cylinder should always be /05ml 1000 ml graduated cylinder markings are 10ml apart, meaning measurements using this device should be reported to the ones place (ex 135ml) The uncertainty value for this volume graduated cylinder should always be /5ml A handson lab from the AACT LibraryHigh uncertainty = not very reliable measurement Accuracy of measurements
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How works Test new features Press Copyright Contact us Creators The graduated cylinder is measured by millimeters, but there is an additional mark that divides each milliliter in half In other words, between 5 and 10 milliliters, there would be 4 markings Is the uncertainty recorded to the tenths or the hundredths place?The uncertainty in mass cylinder comes from the balance reading Put enough water to cover the metal cylinder into a 100mL graduated cylinder and record the volume The graduated cylinder is not very precise;
In the graduated cylinder shown in Figure 1, the mL graduations are marked and can be read with certaintyJ S Fritz and G H Schenk, Quantitative Analytical Chemistry, 3rd ed, Allyn & Bacon, Boston, 1974, p 560Nalgene™ Graduated Cylinder Variety Packs include one each 10mL, 25mL, 50mL, 100mL, 250mL, 500mL and 1000mL cylinders All sizes have a generous pour spout and moldedin graduations that are inked for easy reading No meniscus allows accurate readings every time The large base keeps cylinders
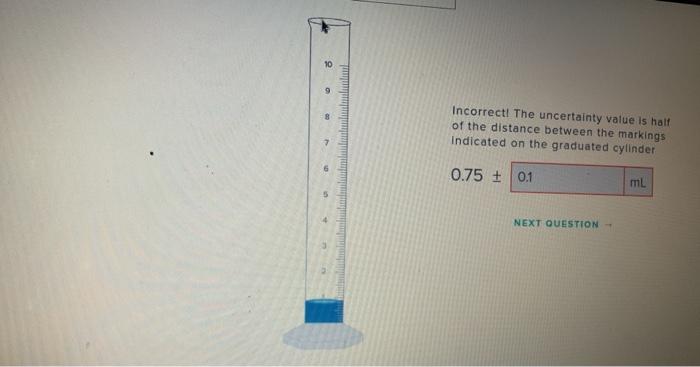



10 9 Incorrect The Uncertainty Value Is Half Of The Chegg Com



C Uncertainty In Measurement Significant Figures
The uncertainty of the 100mL graduated cylinder was ±5mL while the 10mL graduated cylinder has an uncertainty of ±01mL 3 Based on the results, the balance was more accurate This is known because the average deviation for the 10mL graduated cylinder was 087mL and for the 100mL graduated cylinder it was 049mL while for the g weight onFind Z MSDS, related peerreviewed papers, technical documents, similar products & more at SigmaAldrich1 comment share save hide report 100% Upvoted Log in or sign up to leave a comment Log In Sign Up Sort by new (suggested) no comments yet




Pyrex Low Form Griffin Beakers Fisher Scientific



Classroom Resources Measuring Volume ct
Nalgene® graduated cylinders volume 1000 mL, accuracy 50 mL, polypropylene; 1 Examine the 25 mL graduated cylinder provided to you and determine the smallest increment marked Record this increment in your data table 2 Examine the 1000 mL graduated cylinder at its designated station in the lab and determine the smallest increment marked Record this increment in your data tableOf the liquid in Figure 1 is 237 mL In the graduated cylinder shown in Figure 1, the mL graduations are marked and can be read with certainty All graduated glassware is read with one estimated digit, so this measurement is recorded correctly to the nearest 01 mL, with an understood uncertainty of ± 01 mL Figure 1 Reading a Graduated




1pc 500ml Profession Graduated Glass Measuring Cylinder Chemistry Lab Spout Measure Glassware Measuring Cylinder Glass Measuring Cylinderglass Measure Aliexpress




Pyrex 1000 00 Glass Beaker 00ml
Therefore 10mL= (01mL/2) is an uncertainty of 005mL 100mL=(1mL/2) is an uncertainty of 05 mL Another way to think of it is that there are ten 10mL cylinders in an 100mL cylinder, so the 100mL• Graduated Cylinder Method using standard 500 ml and 1000 ml cylinders depending on sample size • Centrifuge Method (ASTM D 4007) using standard 100 ml crude samples and calibrated, 8" conical shaped tubes (with 1% minimum increment);With a 50mL graduated cylinder, read and record the volume to the nearest 01 mL The 10mL graduated cylinder scale is read to the nearest 001 mL and the 500mL graduated cylinder scale is read to the nearest milliliter (1 mL) A buret is a scaled



Classroom Resources Measuring Volume ct




Measuring Cylinder Chemical Laboratory Rs 515 Piece Tmc Engineering Private Limited Id
Once the balloon is completely submerged the reading on the graduated cylinder is 594 ml I estimate that my uncertainty on the volume readings is / 2 ml While submerged I attach the balloon to a digital pressure gauge which records the pressure as being 167; Use the 10 mL graduated cylinder, which will be accurate to two significant figures Mathematical operations are carried out using all the digits given and then rounding the final result to the correct number of significant figures to obtain a reasonable answerKilo‐ k 1,000 hecto‐ h 100 deca‐ da 10 deci‐ d 01 •Graduated cylinders mL 50 mL 05mL 500 mL 10 mL 100 mL THE DAILY NEWS Accuracy or Precision?




Pyrex Glass Griffin Beaker Low Form Measuring 250 Ml Carolina Com




50 Ml Graduated Cylinder Class A At Thomas Scientific
Answer and Explanation The graduated cylinder with the most subdivisions between the mL marks is the most precise Typically this would be the 50 mL graduated cylinder Consequently, what is the uncertainty of a 50 mL graduated cylinder?Suppose we're measuring the volume of water in a 50mL graduated cylinder with markings every 1 mL We examine the meniscus and decide it is closest to 42 mL What we really mean by this statement is that the meniscus is closer to 42 than it is to either 41 or 43, implying that the actual volume lies somewhere between 415 and 425 mL A ruleHow to measure liquid volume with accuracy using graduated cylinders




Laboratory Equipment Lab Pro Inc



Classroom Resources Measuring Volume ct
View Volume and Density Lab2 sheetdocx from PHYS 1410 at Houston Community College CHEM 1405 Lab 2 Volumetric Glassware and Density Determinations Objectives To study volumetric glasswarePipette ±001 mL Burette ±002 mL per reading* *This means that a titre would be recorded as, for example, (28 ± 004) mL 250 mL standard flask ±025 mL 10 mL measuring cylinder ±01 mL 100 mL measuring cylinder ±05 mL 1000 mL measuring cylinder ±5 mL 2 A classification of experimental errors Systematic errorsScience Lab Tabloid since 1630 Accuracy the nearness of a measure to the true or accepted value 1 measurement




Lab 1 Experiment 2 Instrumental Measurements Objectives To Obtain Measurements Of Length Mass Volume And Temperature To Determine The Mass And Volume Of An Unknown Rectangular Solid To Gain Proficiency In Using The Following




Kartell Graduated Cylinders Pmp Vwr
In the graduated cylinder shown in Figure 1, the mL graduations are marked and can be read with certainty All graduated glassware is read with one estimated digit, so this measurement is recorded correctly to the nearest 01 mL, with an understood uncertainty of ± 01 mLALE Uncertainty Name _____ M Davis Page 3 Uncertainty in Measuring Volumes of liquids When you measure liquids in the chemistry laboratory, the volume of the liquid is usually measured in liters (L) or milliliters (mL) A graduated cylinderReading volume on the graduated pipette (or burette) 14 mL Meniscus surface is in fact a little bit below the 14 mL mark, so you may read it as 142 mL, assuming it is about 1/5 of the scale distance So called Schellbach burettes have additional thin, colored line embedded in the glass



Solved Above Is An Image Of A 50 Ml Graduated Cylinder Calculate The Approximate Uncertainty Or Error In In Units Of Ml For Measuring 10 Ml Using T Course Hero



Www Pacificlab Com Au Media Files Brand Volumetric Instruments Selection Pacificlab Pdf
To measure the volume of liquid in a graduated cylinder, you should make a reading at the bottom of the meniscus, the lowest point on the curved surface of the liquid Figure 1 To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 mL marks into tenths of a milliliter, and The 10ml graduated cylinder used had an uncertainty of 10 1 ml In the original measurement of water in the graduated cylinder of 8 75ml there would be a 1 14% uncertainty The yellow digital thermometer had an uncertainty of 10 1 degrees Celsius The thermometer is taking temperatures from 79 5 degrees Celsius to 310 mL graduated cylinder This graduated cylinder has white lines going completely around the cylinder to indicate the mL quantities The shorter lines indicate tenths of a mL With this graduated cylinder, the bottom of the meniscus is more than 8 mL but less than 9 mL One also knows it is greater than 86 mL but less than 87 mL An estimate




Karter Scientific 213c2 Borosilicate 3 Glass Graduated Cylinder 3 Piece Of 10 Sets 50 Ml And 100 Ml 1 Fl Oz Capacity 10 Fl Oz Graduation Interval Amazon Sg Diy Tools




Pyrex 1000 50 Glass Beaker 50ml
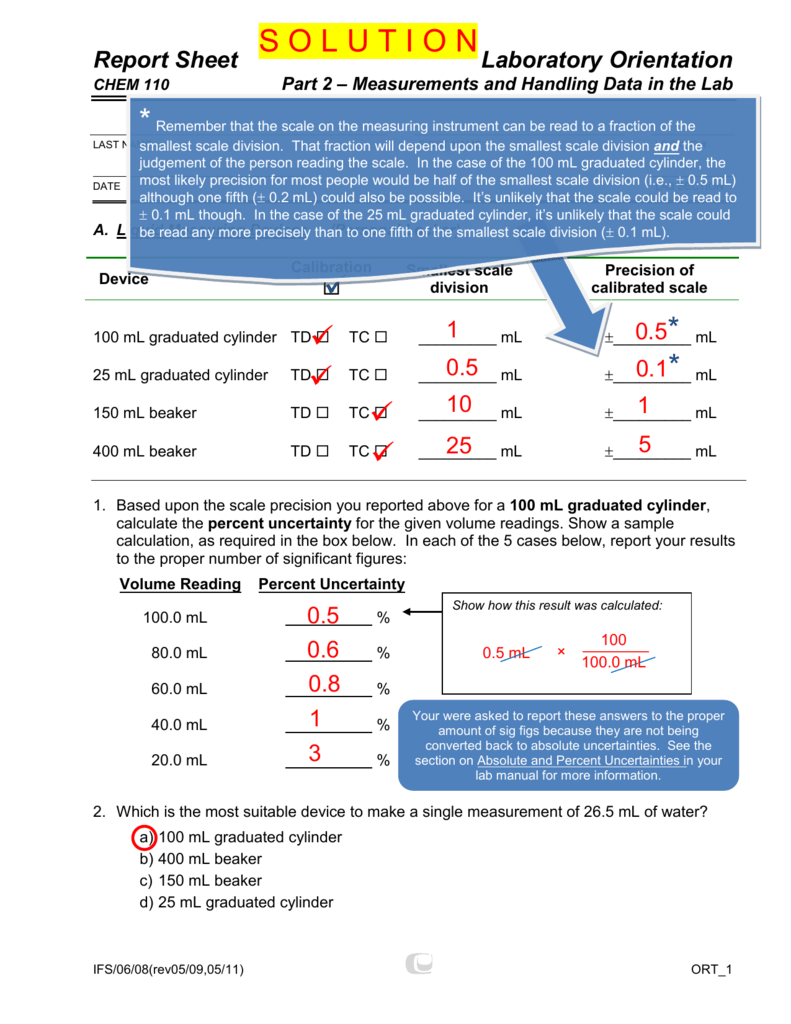
Readings will be ±05 mL (the14 When using a 25mL graduated cylinder to measure 15 mL, the absolute uncertainty is ±1% of 25 mL, or ±025 mL and the relative uncertainty is % 15 02 5 100 17 mL!1000 ml graduated cylinder 2 ml 500 ml graduated cylinder 1 ml 100 ml graduated cylinder 04 ml 10 ml graduated cylinder 008 ml 50 ml buret 010 ml Thermometer with 1° C graduations 05 ° C Thermometer with 02° C graduations 01 ° C Barometer 01 torr



Virtual Lab Measurement 2 Mr Palermo S Flipped Chemistry Classroom




Pyrex Low Form Griffin Beakers Fisher Scientific
Uncertainty An estimate attached to a measurement which characterises the range of values within which the true value is said to lie It is written, for example, as 440 ± 04 Reliability The opposite of uncertainty;ColeParmer elements Product Type Graduated Cylinder $350 $2650USD / Pkg of 1 View All 9 ColeParmer Serialized Class A Graduated Glass Cylinders Calibrated to Deliver View Items to Compare $4100 $ USD / Each ColeParmer Product Type Graduated Cylinder The tolerance on graduated cylinders is about 1% Volumetric flasks, burets and pipets are the most accurate with tolerances of less than 02% Correspondingly, what is the uncertainty of a graduated cylinder?



Www Michigan Gov Documents Mdch Measures Power Point 7 Pdf




Ii Volume Of A Liquid 1 Examine The Four Graduated Chegg Com
DEVICE MEASURES UNIT(S) UNCERTAINTY Glass thermometer Temp °C / 05 °C Metal thermometer Temp /°C 05 °C 10 ml graduated cylinder Volume /ml 005 ml 100 graduated cylinder Volume ml / 05 mlMeasuring Cylinders Plastic graduated cylinders are useful for measuring solutions and liquid levels in a variety of shapes and sizes, ie, 10ml 25ml 50ml 100ml including cylinders that are tall, short, conical, and bellshaped to measure the volume of a liquid These graduated measuring cylinders are fabricated from hard plastics, ceramicsScientific Labware Glass Graduated Cylinder 1000 mL $1999 Bomex Scientific Labware Glass Graduated Cylinder 25 mL Add to cart Scientific Labware Glass Graduated Cylinder 25 mL $299 Bomex Scientific Labware Glass Boiling Flask 500 mL Add to cart Scientific Labware Glass Boiling Flask 500 mL



2




File Glass Graduated Cylinder 250ml 1 Jpg Wikimedia Commons
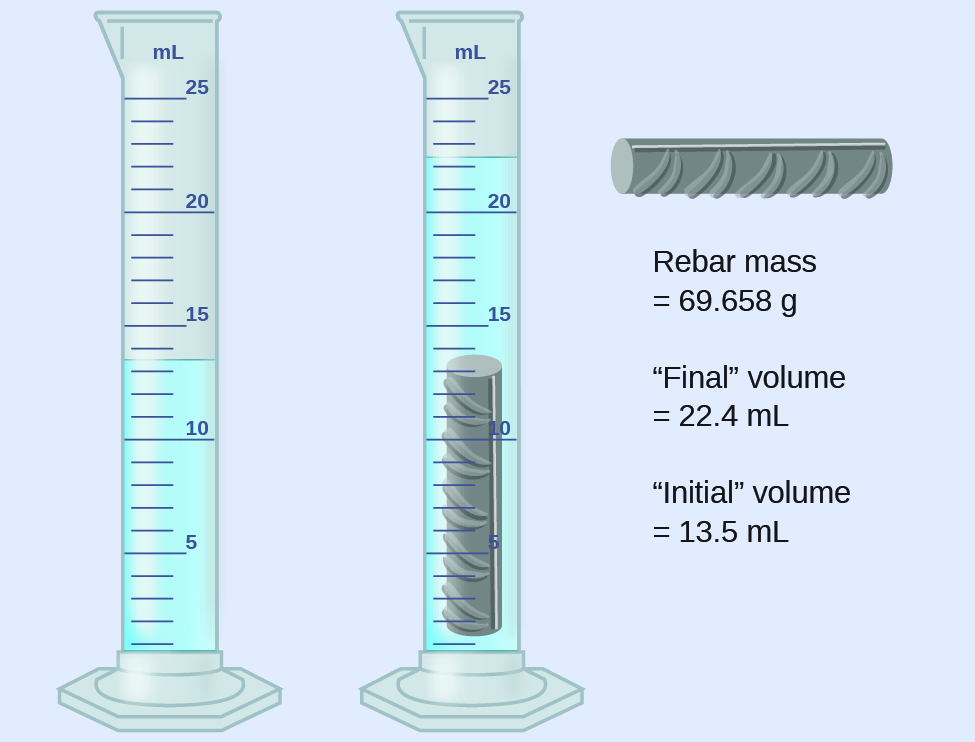
5 Partially filled 10 mL, 100 mL and 1000 mL graduated cylinders will be placed in the front of the classroom Read these cylinders to the correct number of significant figures 6 Obtain a metal block with a hook and string Fill a 500 mL graduated cylinder approximately half full of water and record the volume this is the best way to find uncertainties for everything If you are using something analouge (doesnt work on electricity) you take half the limit of the reading as the uncertainty for example, if the smallest reading on a measuring cylinder is 1 ml, then the uncertainty will be 05 ml for digital instruments, you take the limit of the reading itself as the uncertainty for• Distillation Method (ASTM D 4006) using reduced sample size




Pyrex Vista Beakers Carolina Com




Errors And Uncertainties In Chemistry Internal Assessment The
Nalgene™ Graduated Cylinder Variety Pack Thermo Scientific Nalgene Nalgene™ Graduated Cylinder Variety Packs include one each 10mL, 25mL, 50mL, 100mL, 250mL, 500mL and 1000mL cylinders All sizes have a generous pour spout and moldedExamine the 1000 ml graduated cylinder at its designated station in the lab and determine the smallest increment marked Record this increment in your data table Volume 10 ml 25 mL 100 mL 1000 ml Graduated Cylinder Increment (mL) Uncertainty (mL) Volume of Water (mL) Mass Balance Number Mass of Nickel Trial One Mass of capped, empty vial I have a 10 ml measuring cylinder with a maximum uncertainty of 01 ml and a 100 ml measuring cylinder with a maximum uncertainty of 1 ml I've calculated that using the 10 ml cylinder 7 times would give a 07 maximum uncertainty, or a 07/70=1% percentage uncertainty
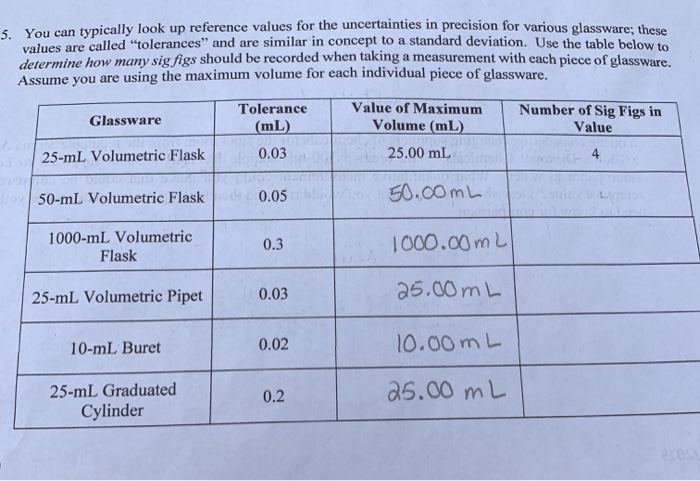



5 You Can Typically Look Up Reference Values For The Chegg Com




Kimble Kimax Griffin Low Form Beakers With Double Scale Graduations All Sizes General Laboratory Supply Inc
1000ml uncertainty Quick question the uncertainty of a 1000ml graduated cylinder would be 10ml right?2Pack 1000ml Plastic Graduated Cylinder, 2Sided Marking Lab Cylinders Clear Science Measuring Cylinder, Test Tube Flask for Home and School Science, Pour Spout $1599 $ 15 99 ($800/Item) Get it as soon as Fri, Jul 30Graduated Cylinders DWK Life Sciences Kimble™ KIMAX™ Class A To Contain Graduated Cylinders Print 1000 mL 10 mL Case for $ Add to cart Accessories View




Graduated Cylinder Wikipedia



Www Pacificlab Com Au Media Files Brand Volumetric Instruments Selection Pacificlab Pdf
Table Precision of Basic Chemical Instruments Instrument Precision balance, platform / 05 g balance, triplebeam / 002 g balance, analytical 150 g / g graduated cylinder, 10 mL / 001 mL graduated cylinder, 50 mL / 05 mL buret, 50 mL / 01 mL pipet, 50 mL / 005 mL volumetric flask, 50 mL / 005 mL volumetric flask, 1000 mL / 030 mL barometer,Question 1 I submerge a small balloon into a large graduated cylinder of water




E8l244 Azlon Measuring Cylinder Squat Form 1000ml Findel International




Glass Beaker 1000ml A B Prospecting



2




Pyrex Glass Graduated Cylinder Class A Works Certified Cylinders Beakers Bottles Cylinders And Glassware Fisher Scientific




Media Portfolio




Pyrex 1000 250 Glass Beaker 250ml




Measurement 100 Ml Graduated Cylinder Units Of Measuring Volume Ppt Download



1




Graduated Cylinders Glassware Plasticware Medical Lab Dental Supplies Healthcare Lab Dental Business Industrial Page 24 Picclick




1 5 Measurement Uncertainty Accuracy And Precision Chemistry




Kimax Graduated Cylinders



Laney Edu Doug Bruce Wp Content Uploads Sites 33 11 12 5 Lab 2 Pdf




Measuring Liquid Volume With A Graduated Cylinder Youtube



1 5 Measurement Uncertainty Accuracy And Precision Chemistry




Read Measurement Of Graduated Cylinder Beaker And Flask Youtube




Thick Glass Graduated Measuring Cylinder Set 5ml 10ml 50ml 100ml Glass With Two Brushes Buy Cheap In An Online Store With Delivery Price Comparison Specifications Photos And Customer Reviews




50 Ml Graduated Cylinder Class A At Thomas Scientific




Nalgene Polypropylene Graduated Cylinders




Why Is Using A Graduated Cylinder More Accurate Than Using A Beaker Socratic




Cp03 Sig Figs Chemteacher




Beakers Low Form Borosilicate Glass Pyrex Vwr




50 Ml Cilindro Graduado Estudiante De Grado Ebay



Www Michigan Gov Documents Mdch Measures Power Point 7 Pdf



1



Laboratory Volumetric Glassware Used In Titration Burette Pipette Astm E287 02 Standard Specification



1




1 2 Uncertainty And A Graduated Cylinder Youtube




Measurement Making Measurements With Accuracy And Precision Ppt Download




Measurement Uncertainty Accuracy And Precision Chem 1305 Introductory Chemistry




Volumetric Flasks Class A Usp Certified



1




Flask Pyrex Volumetric Class A 1000ml
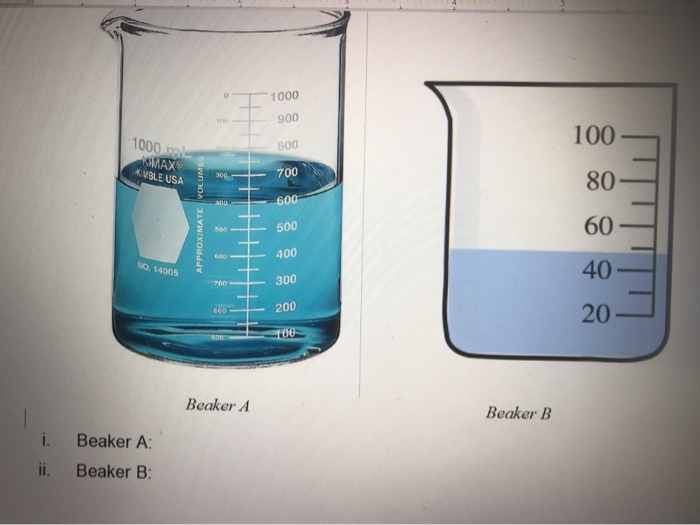



Measure The Liquid In The Beakers Don T Forget To Chegg Com



Blogs Nvcc Edu Alchm Files 16 08 111 02densityandmeasurementsfall16 Pdf




File Plastic Graduated Cylinder 1000ml Jpg Wikimedia Commons




Essential Skills 1



1 9 Essential Skills 1 Chemistry Libretexts




Huaou 250ml Measuring Cylinder With Spout And Graduation With Glass Round Base Laboratory Chemistry Equipment Laboratory Cylinder Aliexpress




Objective The Aim Of This Lab Is To Practice Recording Measurements With The Correct Precision Procedure Homeworklib




Buy 213i13 Karter Scientific 250ml Glass Graduated Cylinder Single Metric Scale In Cheap Price On Alibaba Com



Http Vcechemistry Tripod Com Yr12notes Miscel Experierrors Pdf



Beaker Clipart Graduated Cylinder 50 Ml Graduated Cylinder Drawing Hd Png Download 00x4193 Pngfind




Measurement 100 Ml Graduated Cylinder Units Of Measuring Volume Ppt Download




Hybex Erlenmeyer Flasks




Nalgene Polypropylene Graduated Cylinders



Significant Figures Chemclass Ol Org



Www Michigan Gov Documents Mdch Measures Power Point 7 Pdf



Learn Maricopa Edu Courses Files Download Wrap 1




Solved Examine The Graduated Cylinders Ml 100 Ml An Chegg Com




Buy Pyrex Glass Graduated Cylinder Single Metric Scale 10 Ml Case Of 24 In Cheap Price On Alibaba Com




Seoh Glass Graduated Cylinder 5ml Science Lab Graduated Cylinders Amazon Com Industrial Scientific



Accounts Smccd Edu Batesa Chem210 Labmanual Experiments Exp 01 determination of density Pdf




Reading Graduated Cylinders And Uncertainty Youtube




Pyrex 1000 1000 Glass Beaker 1000ml




Pyrex 1000 600 Glass Beaker 600ml




Amazon Com 500ml Beaker Low Form Griffin Borosilicate 3 3 Glass Double Scale Graduated Karter Scientific 213d26 Kitchen Dining




1000 1000 Ml Printed Graduated Cylinder Pp Case Of 6




Pyrex Glass Griffin Beaker Low Form Measuring 400 Ml Carolina Com




Measuring Volume Of Liquids Most Common Apparatus Measuring Cylinder Comes In Different Sizes E G 10 Ml 25 Ml 50 Ml Ppt Download



Www Vitlab Com Fileadmin User Upload Vitlab Gmbh Sonstiges Druckschriften Broschueren Volumenmessung Pdf Broschuere Volumenmessung En Pdf



Significant Figures Chemclass Ol Org



Www Vitlab Com Fileadmin User Upload Vitlab Gmbh Sonstiges Druckschriften Broschueren Volumenmessung Pdf Broschuere Volumenmessung En Pdf




Strengths And Purposes Of Graduated Cylinders And Beakers Blog




Measuring With Uncertainties Youtube




500 Ml Graduated Cylinder Glass Density Measuring Cylinder 500ml 500cc Display Glassware




Cheap Graduated Cylinder 10ml Find Graduated Cylinder 10ml Deals On Line At Alibaba Com




Glass Measuring Cylinders At Thomas Scientific




Graduated Cylinder Wikipedia



Mary Jane Simpson Middlebury College Chem 103 Lab Page 2



Laboratory Volumetric Glassware Used In Titration Burette Pipette Astm E287 02 Standard Specification




Prelaboratory Questionis 1 What Is The Volume Of Chegg Com




Class A Graduated Cylinder At Thomas Scientific




Graduated Cylinder 50ml Class A Hexagonal Base Borosilicate Gla Eisco Labs



Pdf Document Capuchem Ca Capu Logo


